How Are Naphthalene Derivatives Used in Surfactant Production?
Nov 14,2025What Role Do Pyrimidine Derivatives Play in Biological Systems?
Nov 07,2025Thiophene Derivatives: Uses, Properties, and Applications
Oct 31,2025How Do Triazine Derivatives Act as Antimicrobial or Antifungal Agents?
Oct 24,2025What Makes Carbazole Derivatives Chemically Stable?
Oct 17,2025Carbazole derivatives are a fascinating class of organic compounds widely used in materials science, pharmaceuticals, and electronics. One of the most remarkable characteristics of these compounds is their chemical stability, which makes them highly versatile in various applications. Understanding what contributes to this stability is crucial for researchers, chemists, and engineers who work with carbazole derivatives.
Carbazole derivatives are molecules based on the carbazole core, a tricyclic aromatic structure consisting of two benzene rings fused on either side of a five-membered nitrogen-containing ring. By modifying the carbazole nucleus through substitution at various positions, chemists can obtain a wide range of derivatives with diverse physical, chemical, and electronic properties.
These derivatives are not only valued for their functional versatility but also for their high resistance to chemical degradation, which makes them suitable for harsh chemical and thermal environments. But what is the root of this stability?
The carbazole nucleus exhibits aromaticity, a property that significantly contributes to chemical stability. The conjugated π-electron system allows electrons to delocalize across the tricyclic framework, distributing charge and lowering the overall energy of the molecule. This delocalization means that carbazole derivatives are less reactive toward many chemical reactions, such as electrophilic substitutions that would destabilize non-aromatic structures.
The nitrogen atom in the central five-membered ring contributes a lone pair of electrons to the aromatic system. This electron donation stabilizes the molecule and makes it less prone to oxidation compared to other nitrogen-containing heterocycles. Substituents attached to the carbazole core can further modulate this electron density, either enhancing stability through electron-donating groups or slightly reducing it with electron-withdrawing groups.
Another factor contributing to stability is the rigid tricyclic structure of carbazole derivatives. Unlike flexible molecules that can easily adopt reactive conformations, the planar and rigid carbazole core resists structural deformation. This rigidity reduces the likelihood of reactions that require significant bending or twisting of bonds, such as certain nucleophilic attacks or ring-opening processes.
Additionally, the rigidity helps preserve the conjugation of the π-electron system, which is essential for maintaining chemical stability and desirable electronic properties.
The chemical stability of carbazole derivatives is highly influenced by the types and positions of substituents on the aromatic rings.
Groups such as methoxy (-OCH₃) or amino (-NH₂) donate electron density into the aromatic system, stabilizing the π-electron cloud and making the derivative less susceptible to electrophilic attack.
Substituents like nitro (-NO₂) or cyano (-CN) slightly decrease electron density, which can sometimes make certain positions more reactive. However, when strategically placed, EWGs can enhance oxidative stability by lowering the HOMO energy level, making the molecule less prone to oxidation.
Bulky substituents near reactive sites can act as steric shields, physically hindering attacks from reactive species. This spatial protection is particularly important in applications like organic electronics, where exposure to oxygen or moisture can compromise material performance.
Carbazole derivatives are not only chemically stable in solution but also resistant to heat and light, which is crucial for materials that operate under extreme conditions.
The aromatic and rigid structure allows carbazole derivatives to withstand high temperatures without undergoing decomposition. The energy required to break the aromatic π-system is substantial, giving these molecules a high thermal threshold.
The conjugated π-electron system absorbs and disperses light energy efficiently, reducing the chance of photochemical degradation. This is why carbazole derivatives are commonly used in OLEDs and other optoelectronic devices, where prolonged exposure to light could otherwise degrade less stable materials.
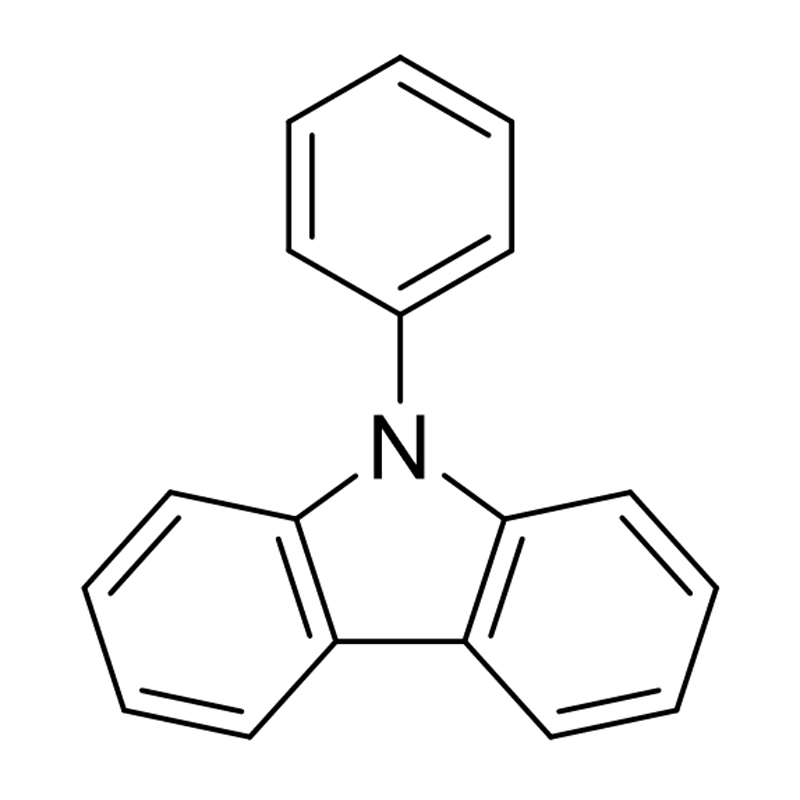
Carbazole derivatives are inherently resistant to oxidation due to the aromatic stabilization of the nitrogen lone pair. However, the degree of resistance depends on substitution:
This property is vital in electronic and pharmaceutical applications, where long-term stability is required.
Solubility and interaction with the environment also influence chemical stability. Carbazole derivatives are generally less reactive in nonpolar solvents, which reduces the risk of hydrolysis or unwanted reactions. In polar or protic solvents, careful selection of substituents can maintain stability while enabling desired solubility.
Furthermore, carbazole derivatives often exhibit resistance to moisture, air, and common acids/bases, making them versatile for industrial applications.
The chemical stability of carbazole derivatives underpins their widespread use:
The remarkable chemical stability of carbazole derivatives arises from a combination of factors:
Understanding these factors allows chemists to design carbazole derivatives tailored for specific applications, whether in electronics, pharmaceuticals, or advanced materials. Their stability is not accidental—it is a product of careful molecular architecture and thoughtful chemical engineering, making carbazole derivatives a cornerstone of modern functional chemistry.
Copyright © 2023 Suzhou Fenghua New Material Technology Co., Ltd. All Rights Reserved.
Custom OLED Material Intermediate Manufacturers

